
| n = p-2 | n = p-1 | n = p | n = p+1 | n = p+2 |
|
H 001 99.985% Stable |
n | |||
|
H 002 0.015% Stable |
||||
|
He 003 0.000137% Stable |
H 003 12.33 yr |
|||
|
Li 004 |
He 004
99.999863% Stable |
H 004 |
|
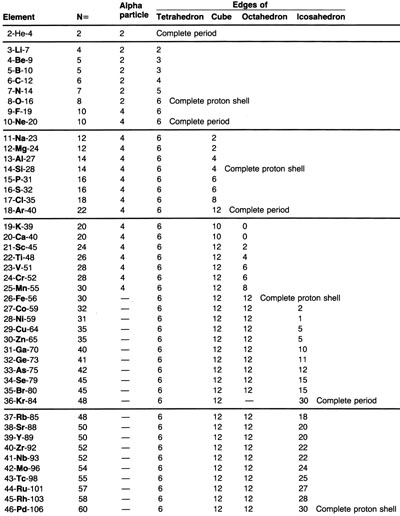
Above and at left you find four varient charts in HTML format. Nuclide
identities displayed in this fashion comprise The Incremental Table of
Atomic Isobars.
| |||||||||||||||||||||||||||||||||||||||||||||
|
Cube Octahedral Equation Icosahedral Equation Our discussion left off examining the isobar-8 so we pick up on isobar-9 which does have a stable nuclide. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
We still have the issue of the cube at hand but the rolly/floppy trait is
mitagated, again by the addition of a central nucleon as was the case
with isobar-7. Hence Be-9 is the only stable isotope of Beryllium. All this makes much sence in terms of both close packing and spherical division. If I were to have you guess which isotope of Boron is the most common B-10 or B-11 what information would you use to guide your choice. Observe to your left, the nuclides Li-7, Be-9, and B-11 are marked off with orange, this denotes them as Most Common Isotope. The yellow is used to mark off the nuclides as SUPER Stable for the six most abundant elements. I consider these six isobars as durable beyond stable and favored by nature. The forth most plentiful element is Neon, we will add that now. the fifth and sixth are already on the the chart, Carbon and Nitrogen. we will observe what paterns arise and begin our discusion anew. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||