Nucleonics Matrix
Nuclear Stability
By Alain Lareau
To begin the Essay on Nuclear stability I wish you to examine what the three most abundant elements are.
That is to ask; after hydrogen and helium which element is the most plentiful in the cosmos?
Atomic
Number | Name | Symbol |
|---|
| 1 | Hydrogen | H |
| 2 | Helium | He |
We have a fair understanding of physical substance by the virtue of chemical interaction at the atomic level. We have a basis in our understanding in our ability to discern a periodicisity of atomic interplay mapped to weight. Thus the Periodic Table and a means to predict associated interplay of energy. We see geometry at work with electron configuration, there is no reason we should not see it with nucleons as well.
We first study both of the two stable isotopes of Hydrogen and Helium which comprise the isobars 1 though 4
| n =
|
| p-2 |
p-1 | p |
p+1 | p+2 |
| |
H 1 |
| | |
| | |
H 2 |
| |
| |
He 3 |
| | |
| | |
He 4 |
| |
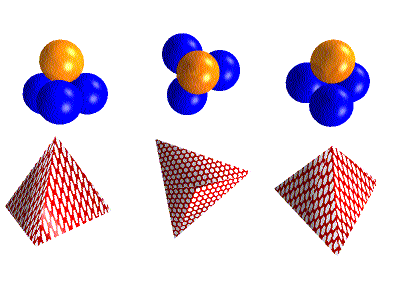
There is a shape in 3D space called the Tetrahedron that when we see it
can help us make sence of two things(ideas). One is that a sphere can
be split into four equal parts the other close packing of four
equal balls. There is a lot to say about this and the Tetrahedron itself.
Use these two links if you wish to investigate this.
Tetrahedron
TetrahedralEquation
| n =
|
| p-2 |
p-1 | p |
p+1 | p+2 |
| |
H 1 |
|
n |
|
| | |
H 2 |
| |
| |
He 3 |
|
H 3 |
|
| | |
He 4 |
| |
| n = p-2 |
n = p-1 |
n = p |
n = p+1 |
n = p+2 |
| |
H 001
99.985%
Stable
|
|
n |
|
| |
|
H 002
0.015%
Stable
|
|
|
| |
He 003
0.000137%
Stable
|
|
H 003
12.33 yr
|
|
Li 004
|
|
He 004
99.999863%
Stable
|
|
H 004
|
As you progress through this web site charts will be made longer and/or
wider and provisions are made for you to add data to these cells as you
see fit.
Charts will be displayed in a sequencial format when posible, for instance:
begining with the most common isotope
first the elements most common in the cosmos
second the elements common in the earth's crust will be added
then the most common salts of seawater
the makeup of our atmosphere next
all stable isotopes of these elements will then be viewed
elements of biological systems if not already included
next all isotopes of half-life periods longer than one day
then metals just heavier than iron will be included
all naturaly occuring radioactive isotopes
all chemicaly significant elements
untill finally all elements and all man made isotopes.